FDAzilla The Leading Platform for GMP Inspection Analytics
OVERVIEW
FDAZILLA.COM TRAFFIC
Date Range
Date Range
Date Range
FDAZILLA.COM HISTORY
WEBPAGE AGE
LINKS TO FDAZILLA.COM
The FDAzilla family of products unlocks regulatory insights and reduces risk for FDA regulated businesses. Expert Guidance from Barb Unger. Read the Medical Device Blog. Week of Mar 4th 2018 FDA Sent These Warning Letters to Pharma Companies. CGMP Quality Assurance and FDA 483s. 74 New FDA 483s March 15th 2018.
ASSOC DIR FOR COMM SCIENCE.
The process of approving these medical devices is flawed according to the Institute of Medicine. All clinical trials must report.
Aberdeen Area Office - Facility Engineer. AIH - Contract Health Clerk. Business Office - Billing Technician. Facility - Boiler Plant Operator.
Branch Chief Grants Management Program. Developmental Therapeutics Branch CCR NCI.
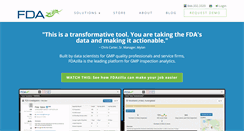
WHAT DOES FDAZILLA.COM LOOK LIKE?


CONTACTS
WHOISGUARD, INC.
WHOISGUARD PROTECTED
P.O. BOX 0823-03411
PANAMA, PANAMA, 00000
PA
FDAZILLA.COM SERVER
NAME SERVERS
FAVICON

SERVER SOFTWARE AND ENCODING
We caught that this website is operating the Microsoft-IIS/8.5 operating system.SITE TITLE
FDAzilla The Leading Platform for GMP Inspection AnalyticsDESCRIPTION
FDAzilla provides actionable information on FDA-regulated companies to pharmaceutical, medical device, food, financial professional services companies.PARSED CONTENT
The web page fdazilla.com had the following in the web site, "The FDAzilla family of products unlocks regulatory insights and reduces risk for FDA regulated businesses." I saw that the website stated " This is a transformative tool." They also said " You are taking the FDAs data and making it actionable. Built by data scientists for GMP quality professionals and service firms, FDAzilla is the leading platform for GMP inspection analytics. Sign up for a demo. Data Integrity The Whole Story. China Drug Inspections, 2016. 10 Ways to Prep for a GMP Inspection. Sign up for a demo."SEE SUBSEQUENT WEBSITES
Für weitere Informationen wenden Sie sich bitte an Dr.
Die FDB ist der technische Fachverband für den konstruktiven Betonfertigteilbau. Sie vertritt die Interessen ihrer Mitglieder national und international und leistet übergeordnete Facharbeit in allen wesentlichen Bereichen der Technik. Lehrgang Betonfertigteilexperte im Januar 2018.
Go to the page content. A3 JURIS Huissiers de Justice. Déposer ou consulter un règlement en un seul clic! Pour connaître tous nos tarifs. Toutes nos coordonnées, où nous trouver .